Medical device-related pressure injury prevention: What caregivers need to know
Build awareness of device dangers and protect patients from skin breakdown.
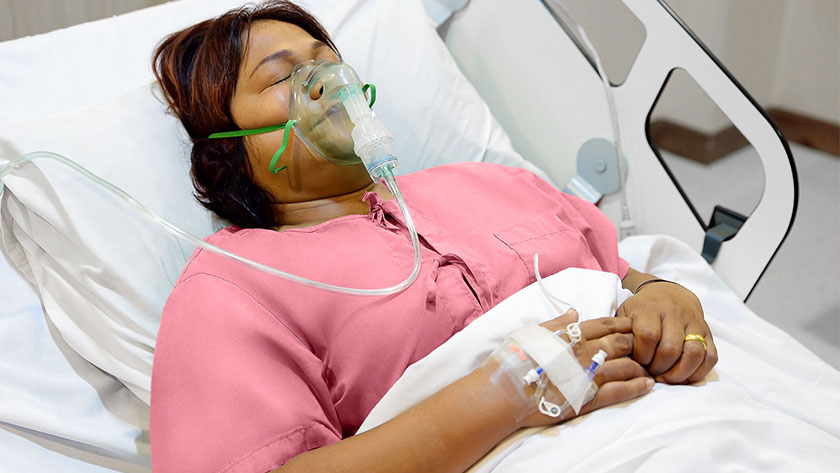
For caregivers, the peak of the COVID-19 pandemic was a reminder that life-saving medical devices can also cause pressure injuries. That’s because critically ill patients with COVID-19-related acute respiratory distress syndrome often require mechanical ventilation, contributing to the formation of medical-device related pressure injuries (MDRPIs). It’s an unfortunate trade-off that the same medical devices that promote healing can also harm skin integrity—but facilities can include these devices in their pressure injury (PI) prevention protocols to reduce the risk of MDRPIs.
29%
of hospital-acquired pressure injuries (HAPIs) are related to medical devices.1
Know the equipment that commonly contributes to a medical device-related pressure injury
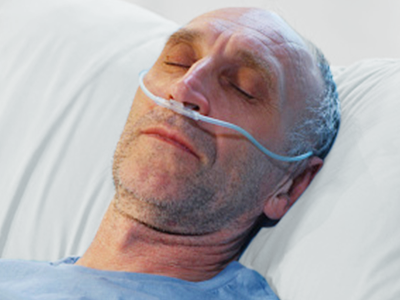
Tubing devices such as oxygen tubing
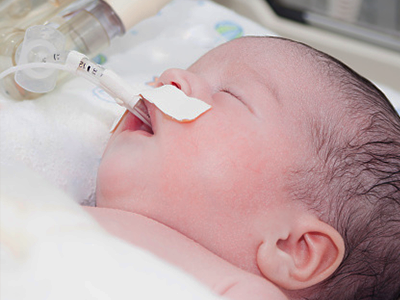
Nasogastric tubes and endotracheal tubes
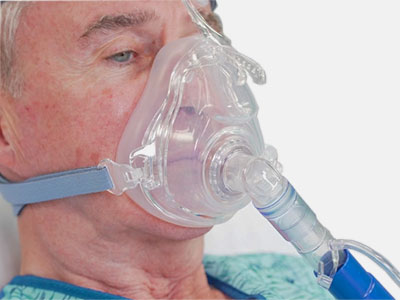
Respiratory masks including CPAP
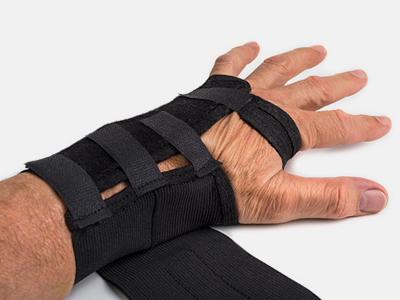
Splints
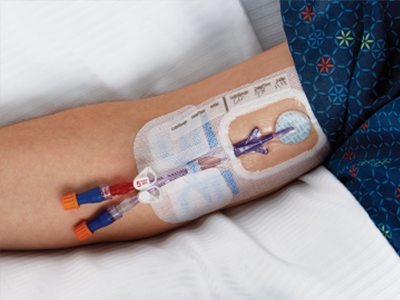
Intravenous catheters
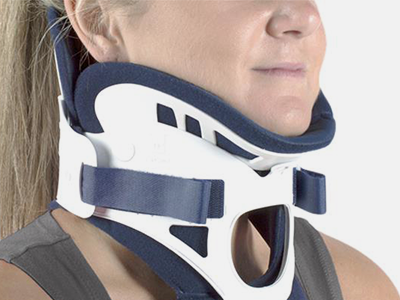
Cervical collars
Research has shown that the risk factors for developing a MDRPI are about the same as for any pressure injury. In other words, just as every patient is considered at risk for a pressure injury, every patient with a medical device is at risk for an MDRPI.
With the right best practice guidance, education and training and system of PI prevention products, you can help empower staff to reduce the risk of medical device-related pressure injuries.
Build caregiver awareness of the hidden dangers of medical devices
It’s not just critical care patients that you need to worry about when it comes to device-related injury. “The term ‘medical device-related pressure injury’ is used in an exponentially growing volume of scientific papers that analyze the wide impact of MDRPIs on nearly all types of care facilities, from operating rooms to rehabilitation,” notes Amit Gefen, PhD, Professor of Biomedical Engineering, Tel Aviv University, and Medline consultant.
Dr. Gefen extensively researched MDRPIs and led work on a consensus document to help clinicians understand and avoid them. He says, “One reason MDRPIs are difficult to prevent is because they’re caused by devices that were designed long before good understanding of the etiology of PIs existed.”
Until device engineering meets current knowledge, there are still many ways caregivers can help protect patients. The list below gives a head start on building awareness of the hidden dangers of medical devices.
“One reason MDRPIs are difficult to prevent is because they’re caused by devices that were designed long before good understanding of the etiology of PIs existed.”

Amit Gefen
PhD, Professor of Biomedical Engineering, Tel Aviv University, Medline consultant
8 considerations of medical device-related pressure injuries
It makes sense to make medical device-related pressure injuries part of your PI prevention protocols. But it’s important to remember that this type of skin breakdown comes with some distinct characteristics and challenges.
Keep these considerations in mind:
- Formation speed. MDRPIs typically develop faster than other common pressure injuries because the devices make it hard to see and assess the skin.
- Risk areas. Critically ill patients may have multiple medical devices, placed in areas where you don’t normally see pressure injuries.
- Effect on microclimate. Humidity and heat that develop under a device can change the skin’s microclimate, making skin more susceptible to negative effects of friction. A prime culprit: respiratory devices.
- Attachment methods. Medical devices that need to be secured tightly to do their job properly can increase pressure on fragile skin.
- Patient sensory issues. Patients who are sedated, immobile or can’t feel the pain a device may cause can be at greater risk of injury.
- Generic designs. Common one-size-fits-all designs don’t fit everyone, especially infants and young children.
- Design obstacles. Medical devices are often made of stiff material that can cause skin to distort. In addition, products used to secure medical devices, such as tape and straps, may make it hard to assess underlying skin.
- More than medical. Be sure to watch out for non-medical devices, such as cell phones, jewelry, glasses, hearing aids, and hairbrushes or combs. These can also contribute to pressure injuries.
As the understanding of device-related pressure injuries grows, prevention strategies have also improved, Dr. Gefen explains. Read on to discover ways to help minimize the risk of MDRPIs.
The key to keeping medical device-related pressure injuries at bay? Routine skin assessments.
Skin assessments are a critical part of every patient’s journey through both acute and post-acute care settings. During these routine assessments, be sure to check under and around medical devices whenever possible.
By lifting a medical device, you do two things: 1) You take away some of the pressure for a moment and 2) you may catch a pressure-related injury early, giving you the opportunity to adjust the device or to implement treatment strategies.
Ongoing skin assessments are especially important with newborns and infants because a medical device could easily cover a large percentage of their body. Also, newborn and infant skin is thinner and more sensitive to irritation than adult skin, and therefore more vulnerable to skin breakdown.
What leading authorities are saying about MDRPI prevention
The National Pressure Injury Advisory Panel recognized that MDRPIs are a distinct subset of PIs. They developed a series of educational posters to help staff follow best practices in reducing the risk of these injuries. The posters break down MDRPI prevention into four patient categories: general population, critical care, pediatric and long-term care.
Here’s a summary of MDRPI prevention from NPIAP:
- Follow manufacturer instructions for each device
- Secure devices without creating additional pressure
- Remove medical devices as soon as possible
- Cleanse and dry the skin under medical devices
- Reposition patients or adjust the device to redistribute pressure and decrease shear
- Avoid placing a medical device under a patient
- Use support surfaces and accessories to help reduce pressure and shear
- Consider using a prophylactic dressing that can help manage friction, shear and microclimate
- Avoid placing a device over an existing pressure injury or damaged skin
One more tip: A skin champion team can help you spread the word and educate frontline staff about MDRPI prevention. Skin champions can be valuable when training new and traveling staff, because other healthcare organizations may use devices produced by different companies. Although created for the same purpose, different design and material may affect skin differently.
Learn more about MDRPIs with these two leading resources
The international consensus document Device-related pressure ulcers: SECURE prevention, analyzes the scale of the problem of MDRPIs, their pathophysiology, and how to best prevent or treat them.
Access MDRPI education from the National Pressure Injury Advisory Panel.
Key takeaway
Medical devices that help patients may also contribute to the formation of pressure injuries. It’s important to understand how medical—and sometimes non-medical—device-related pressure injuries can form and how to prevent them by refocusing on the basics. Conducting proper skin assessments, following best practices and educating your team on MDRPIs can help you keep patients safe.
References:
- Kayser, S. A., VanGilder, C. A., Ayello, E. A., & Lachenbruch, C. (2018). Prevalence and Analysis of Medical Device-Related Pressure Injuries: Results from the International Pressure Ulcer Prevalence Survey. Advances in Skin & Wound Care, 31(6), 276–285. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5991189/
- Black, J. M., Cuddigan, J. E., Walko, M. A., Didier, L. A., Lander, M. J., & Kelpe, M. R. (2010). Medical device related pressure ulcers in hospitalized patients. International Wound Journal, 7(5), 358–365. Available at https://doi.org/10.1111/j.1742-481x.2010.00699.x
- Gefen, A., Alves, P., Ciprandi, G., Coyer, F., Milne, C. T., Ousey, K., Ohura, N., Waters, N., Worsley, P., Black, J., Barakat-Johnson, M., Beeckman, D., Fletcher, J., Kirkland-Kyhn, H., Lahmann, N. A., Moore, Z., Payan, Y., & Schlüer, A. B. (2020). Device-related pressure ulcers: SECURE prevention. Journal of Wound Care, 29(Sup2a), S1–S52. Available at https://www.magonlinelibrary.com/doi/full/10.12968/jowc.2020.29.Sup2a.S1
- Blume-Peytavi, U., & Kanti, V. (2018). Prevention and treatment of diaper dermatitis. Pediatric Dermatology, 35, s19–s23. Available at https://doi.org/10.1111/pde.13495
- Sleiwah, A., Nair, G., Mughal, M., Lancaster, K., & Ahmad, I. (2020). Perioral pressure ulcers in patients with COVID-19 requiring invasive mechanical ventilation. European Journal of Plastic Surgery, 43(6), 727–732. Available at https://doi.org/10.1007/s00238-020-01737-6