Iodine for wounds: Why it’s better for biofilm management
Discover the benefits of using iodine on wounds and how it advances healing.
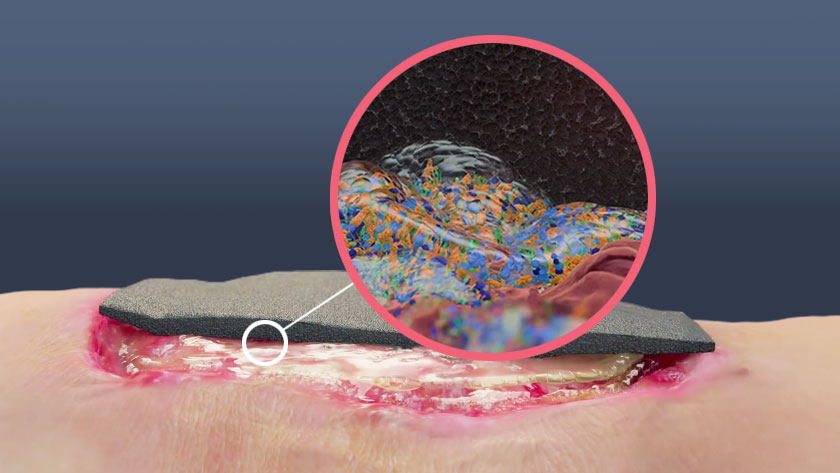
When your patient is suffering from a stalled wound, it may be due to biofilm in the wound. Biofilm poses a significant obstacle to healing and has been shown to be present in up to 90% of chronic wounds and 6% of acute wounds.1
Research shows that the key to reducing biofilm in order to help chronic wounds heal is a combination of therapies as part of an aggressive “Step down, step up” clinical method.2 Our poster can help your team implement this strategy to biofilm management.
Antibacterial dressings are part of this approach, but with so many options, it can be difficult to know what to choose.
What’s the only antibacterial dressing that effectively reduces bioburden?
There are antibacterial dressings that incorporate a range of ingredients—from iodine to silver to gentian violet to methylene blue—so it’s important to know the right intervention to choose.
When you encounter a hard-to-heal wound with biofilm, iodine is proven to help. “Unlike silver or gentian violet and methylene blue, iodine more effectively reduces bioburden in the wound dressing,” explains Patricia Turner, BSN, RN, CWOCN, CWS, Medline Director of Clinical Resources, Skin Health Acute Care.
“Wounds respond rapidly to iodine,” Turner says. “And, with the barriers to healing reduced, clinicians can move onto advanced therapies such as collagen or negative pressure wound therapy.”
Slow-release iodine for wounds: A safe, gentle approach to healing
You probably recognize iodine as an antiseptic used to prepare patient’s skin before surgery, but you may wonder, “Is iodine good for healing wounds?” Yes, few antibacterial dressings have the long history and evidence-based results that iodine does.
Although early concerns about iodine focused on the pain and irritation it sometimes caused, this can be avoided with an iodophor foam dressing. These dressings contain iodophors, which combine iodine with a stabilizing agent designed to release iodine in a gentle, controlled manner and maintain efficacy against bacteria. “In a slow-release dressing, the iodophor makes delivery safe,” Turner says.
“In a slow-release dressing, the iodophor makes delivery safe.”

Patricia Turner
BSN, RN, CWOCN, CWS, Medline Director of Clinical Resources, Skin Health-Acute Care.
There are three types of iodophors used in wound dressings in the US:
- Cadexomer iodine: produced by the reaction of dextrin with epichlorohydrin and iodine.
- Povidone iodine: a liquid chemical complex of povidone, hydrogen iodide and elemental iodine.
- Absorptive PVA-based foam dressing: a newer iodophor—the exact chemical structure of which has not yet been fully determined—which is specifically indicated for use on infected wounds.
The iodophor ensures the release of iodine in a controlled manner. These formulations reduce the chance of “dose dumping” of the active iodine because it’s released “on demand.” 3 In other words, the iodophors create an equilibrium, releasing more iodine as the dressing absorbs more fluid. In this way, the dressings achieve safe delivery.
More on dose dumping
Dumping large quantities of iodine in a wound is, unfortunately, a commonly used approach. This can be toxic to skin tissues and does not deliver a sustained antibiotic effect over time. Just like with oral antibiotics, iodine needs to be delivered to the wound regularly over a sustained period of time—typically seven to 10 days—to be effective. That’s why slow-release technology is key.
Unlocking the evidence for iodine on wounds
When it comes to choosing an effective antibacterial dressing for biofilm management, it helps to look at the evidence. “Evidence shows that iodine is the most superior, broad spectrum antibacterial agent available,” Turner says, adding “It is effective against bacteria and antibiotic-resistant species.”
Evidence shows that iodine is the most superior, broad spectrum antibacterial agent available.
Ex vivo study
In one study based on an ex vivo porcine skin model, cadexomer iodine demonstrated a 7 log kill of mature three-day P. aeruginosa biofilm after 24 hours and 72 hours of exposure. 4
These 4 in vitro studies show evidence that supports the use of a controlled-release iodine dressing to manage biofilm in the wound:
- More effective, sustained antibacterial effect. A two-layer hydrogel dressing that releases oxygen and iodine in the wound dressing was more effective than other therapies against both 24-hour Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus). The study also revealed that the iodine dressing had a sustained antibacterial effect throughout the treatment period, reducing biofilm levels of each organism to below minimum detection levels after 24 hours.5
- Completely disrupts bacteria. A controlled-release iodine dressing showed complete disruption of the bacteria in a multispecies biofilm after seven days of use.6
- Decreases infection-causing bacteria. An in vitro porcine explant model found that controlled-release iodine dressings decreased mature, three-day P. aeruginosa by eight logs after one and three days.7
- Significant chronic biofilm reduction and cadexomer iodine. In a review of 39 articles that looked at topical agents used for managing chronic biofilm, the results indicated cadexomer iodine had the highest mean log10 reduction of biofilm.8

The proven iodine dressing clinicians rely on
IoPlex® Iodophor Foam Dressing uses slow-release technology to gently and safely deliver iodine to the wound. While several iodine dressings use this technology, the latest cutting-edge iPlexomer® technology consists of an absorptive, PVA-based foam dressing that releases iodine as the dressing absorbs fluids. IoPlex is specifically indicated for infected wounds, an indication of remarkably few advanced dressings.
One in vitro study confirmed its superior disruption of biofilms compared to other antibacterial dressings. The graph below illustrates the study’s results, which used an in vitro CDC bioreactor biofilm model. It showed that, in all cases but one, there was one dressing that outperformed the others. IoPlex performed better than dressings with silver, gentian violet and methylene blue.9
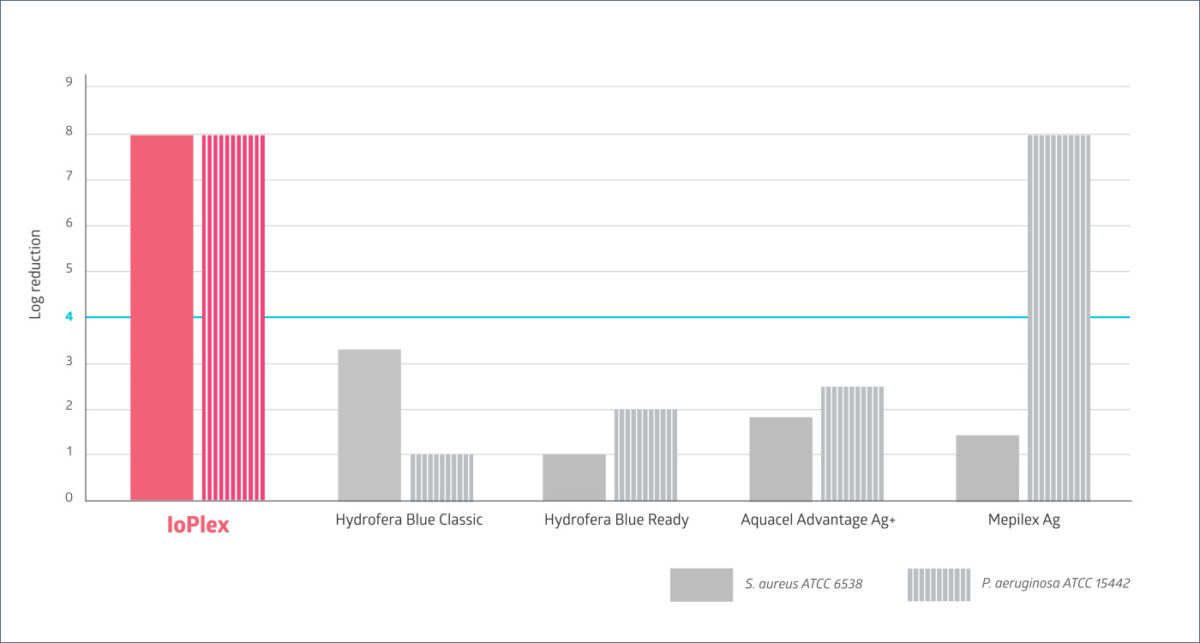
In-vitro testing showed that IoPlex was the only competitive dressing to have a greater than 4 log reduction against both S. aureus and P. aeruginosa biofilm strains. IoPlex has potent activity against biofilms. The clinical significance of these findings has not been determined.
As an added benefit, when the iodine in IoPlex is released, the dressing changes from black to white, which indicates the dressing is ready to be changed. Turner notes, “This reduces confusion and waste, ultimately saving staff time and money.”
Key takeaway
Iodine has been used in medicine for centuries, and current evidence backs up its efficacy as an antibacterial. But it’s key for caregivers to use slow-release technology to deliver iodine to the wound. Dose dumping large quantities of iodine is ineffective, potentially dangerous and not a best practice. That’s why experts recommend a controlled-release iodine dressing as part of a step down, step approach to biofilm management.
References(Accessed Oct. 13, 2020):
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3839004/
- https://onlinelibrary.wiley.com/doi/epdf/10.1111/wrr.12590
- https://onlinelibrary.wiley.com/doi/pdf/10.1111/iwj.12142
- Data on File
- https://pubmed.ncbi.nlm.nih.gov/19862874/
- https://pubmed.ncbi.nlm.nih.gov/20378671/
- https://www.liebertpub.com/doi/pdf/10.1089/9781934854013.299
- https://pubmed.ncbi.nlm.nih.gov/31899281/