Build your business case for electronic hand hygiene monitoring
Gain leadership buy-in with this 3-step process.
As a patient safety strategist, when I share the latest approaches to optimizing hand hygiene with infection preventionists, I typically ask: “Are you responsible for driving hand hygiene compliance improvement?” Usually everyone answers, “yes.”
My follow-up gets the opposite response: “Do you have a discretionary budget and authority to spend it on a vetted technology, product or service you feel will help drive sustainable improvement in compliance?” Usually no one says “yes.” This leads me to conclude that improvements in hand hygiene compliance require very sound arguments to convince leadership to allocate precious organizational resources.
A strategic framework for success
Here is a simple strategic framework to help you make the case for technology adoption and influence organizational leadership to buy-in:
First, be sure you can align your request with organizational priorities, keeping your eye on the best interests of the organization’s community at large.
- Is patient safety, high reliability, getting to zero harm a stated organizational goal? If yes, then align your request with how adoption will support and drive success with these organizational goals.
- How will adoption address obstacles to the ideal future state? Create a vision for achieving the ideal state as the end game that adoption will help realize.
Next, identify all clinical and economic key influencers/decision-making stakeholders and know what is important to them.
- Target each person with simple messages that define how their interests will be served.
- Listen to any barriers/obstacles they raise and collaborate to remove or mitigate them to acceptable levels.
Finally, focus on the math.
- Get the evidence and math right.
A 3-step process
The following three-step process will help you with the numbers:
- Know your baseline: What are you doing today and what does it really cost?
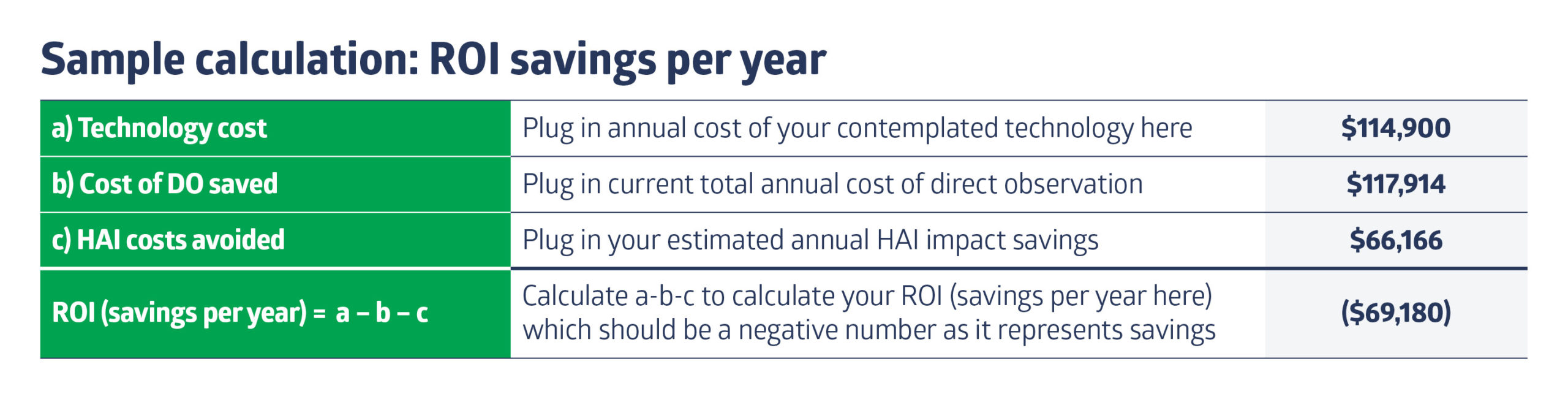
Assuming you are doing direct observation (DO), you can calculate the true cost of DO based on the evidence based-calculation1 as published in my article “Hand hygiene observation: What’s the budget to meet the Leapfrog Group standard?” Using the 383-bed teaching hospital as an example, the total cost for DO for inpatient units was $117,914 per year. - Calculate the cost for the technology.
Let’s say the one you’re looking at costs $300 per bed per year on a subscription basis; that comes out to $114,900 per year (plug in whatever number applies to the technology you are considering; include all costs including any costs for badges, maintenance, repair and battery replacement, if any). - Make a sound assumption for what the impact on healthcare-associated infections (HAIs) will be and calculate the ROI.
Let’s look at two examples where electronic hand hygiene systems were implemented and had a positive impact on MRSA and C. diff rates, as these are infections known to be easily transmitted by the hands. Kelly, et al2 showed a 43% reduction in MRSA infections and estimated each avoided infection saved the hospital $18,083. In this instance, the savings amounted to $434,000 or $670 per bed per year. Robinson, et al3 showed a 66% reduction in the rate of C. diff but did not do any economic impact analysis. Zhang, et al4 estimate the incremental cost of a C. diff infection to be $25,000.
Eliminating just one MRSA and one C. diff infection could save over $43,000 per year. Eliminating two of each would save over $86,000. Using the data from Kelly and Robinson as conservative baselines, how many MRSA and C. diff infections could you avoid by implementing an electronic hand hygiene compliance system (or any innovative technology for which you wish to make the business case)?
Here is an example assuming just two MRSA and one C. diff infections are eliminated (cost avoidance = $61,166 per year).
Calculate the ROI dollars. For this example:
Then, be sure to add in the additional operational benefits, if any, of the technology you are proposing. Examples include:
- Provides accurate data
- Enables feedback to frontline staff
- Capable of real-time reminders at the point of care to prevent possible missed hand hygiene opportunities
- Supports our organizational goals
- Supports an enriched patient safety culture
No matter what technology or solution you are considering, this method for influencing leadership with a well-documented, science-supported business case will help you strategically frame your request in a sound manner consistent with a value-driven, high reliability organization.

VP Patient Safety Innovation for Medline Industries, LP
Paul invented the first electronic hand hygiene monitoring system designed to improve hand hygiene performance while reducing infections and costs. He is an innovative and mission-driven leader with more than 35 years of experience in hand hygiene and patient safety solutions. Paul also shared hand hygiene insights as a past contributor to Healthcare Hygiene Magazine.
References:
- Srigley JA, et al. Quantification of the Hawthorne Effect in Hand Hygiene Compliance Monitoring Using an Electronic Monitoring System: a Retrospective Cohort Study. (2014) BMJ Qual Saf. 23, 974-80.
- Kelly JW, et al. Electronic Hand Hygiene Monitoring as a Tool for Reducing Health Care Associated Methicillin-Resistant Staphylococcus aureus Infection. (2016) Am J Infect Control. 44(8), 956-957.
- Robinson N, et al. Innovative Use of Electronic Hand Hygiene Monitoring to Control a Clostridium Difficile Cluster on a Hematopoietic Stem Cell Transplant Unit. (2014) Am J Infect Control. S29-S166, S150.
- Zhang D, et al. Attributable Healthcare Resource Utilization and Costs for Patients with Primary and Recurrent Clostridium difficile Infection in the United States. Clin Infect Dis 2018.
Disclosure: Medline was a 2021 member of the Leapfrog Partners Advisory Committee and has a collaborative relationship with a company that offers electronic hand hygiene monitoring services.