Choosing isolation gowns: How to know the right barrier protection
Start by understanding AAMI level ratings and the fluid resistance needed.

Whether you work in acute care or long-term care, your facility should have an overall infection prevention strategy to prevent the spread of healthcare-associated infections (HAIs). This strategy includes protocols for personal protective equipment (PPE), which helps keep everyone safe.
Isolation gowns are an important component of PPE, acting as a barrier between frontline workers and potentially infectious liquid and solid material.1 It sounds simple, yet different activities require different levels of protection. Using the right isolation gown will protect your skin and clothing. What level—or multiple levels—of gowns do you and your team need as you provide care for patients or residents in your healthcare facility?
AAMI isolation gown ratings
The Association for the Advancement of Medical Instrumentation (AAMI) classifies isolation gowns according to four levels of protection from fluid penetration. Keep in mind that cover gowns are not rated and should be used for minimal risk situations only, such as in food service, for visitors or for training.
Level 1—Minimal barrier protection
The right gown for minimal-risk situations:
- Provides a slight barrier to small amounts of fluid penetration
- Single test of water impacting the surface of the gown material is conducted to assess barrier protection performance²
For example, use during basic care, standard isolation or contact precaution, or in a standard medical unit.

Level 2—Low barrier protection
The right gown for low-risk situations:
- Provides a barrier to larger amounts of fluid penetration through splatter and some fluid exposure through soaking
- Two tests are conducted to assess barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
For example, use during droplet precaution, blood draw, suturing, in the intensive care unit (ICU) or a pathology lab.

Level 3—Moderate barrier protection
The right gown for moderate-risk situations:
- Provides a barrier to larger amounts of fluid penetration through splatter and more fluid exposure through soaking than Level 2
- Two tests are conducted to test barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
For example, use during droplet precaution, arterial blood draw, inserting an intravenous (IV) line, in the emergency department, in the decontamination/sterile processing department or for trauma cases.

Level 4—High barrier protection
The right gown for high-risk situations:
- Prevents fluid and virus penetration²
- In addition to the other tests conducted under levels 1-3, barrier level performance is tested with a simulated blood containing a virus. If no virus is found at the end of the test, the gown passes.
For example, use during long, fluid-intense procedures, surgery, when pathogen resistance is needed or when highly infectious diseases are suspected (for instance, Ebola, per the Centers for Disease Control and Prevention [CDC]).1
Only Level 4 gowns are considered fluid-impermeable, as opposed to fluid-resistant, with seams and closures that prevent liquid penetration.²
Hazard and risk identification
When determining the appropriate level of gown, you should start by looking at activities that involve touching or being close to patients or long-term care residents. Specifically, identify the pressure and type of contact being administered, the duration and types of tasks performed. If a patient or resident has an infectious disease, make sure care providers know the stage of the disease and the severity of symptoms.3
If you work in a long-term care facility, keep in mind that there are standard and enhanced barrier precautions, each requiring proper gown selection:
- Standard precautions—Infection prevention practices that apply to the care of all residents, regardless of suspected or confirmed infection or colonization status. They are based on the principle that all blood, body fluids, secretions and excretions (except sweat) may contain transmissible infectious agents. These practices apply to any potential exposure to:
- Blood
- Body fluids
- Mucous membranes
- Non-intact skin
- Potentially contaminated environmental surfaces or equipment
- Enhanced barrier precautions—Used during high-contact resident care activities that provide opportunities for transfer of multidrug-resistant organisms (MDROs) to staff hands and clothing:
- Dressing
- Bathing/showering
- Transferring
- Providing hygiene
- Changing linens
- Changing briefs or assisting with toileting
- Device care or use: central line, urinary catheter, feeding tube, tracheostomy/ventilator
- Wound care—any skin opening requiring a dressing4
Once you’ve identified hazard and exposure risks, you and your team can select gowns guided by how protective clothing materials provide protection against microorganisms in blood and body fluids. A microorganism’s movement through protective clothing materials depends upon several factors:
- Physical and chemical properties of the fabric—Includes factors such as thickness, pore size and repellency
- Shape, size and other characteristics of the microorganisms—Includes factors such as form and structure, ability to move and adaptation to environmental extremes
- Characteristics of the carriers—Includes factors such as surface tension, volume and thickness
- External factors—Includes factors such as physical, chemical and thermal stresses
Remember that microorganisms are transported by carriers such as body fluids, sloughed skin cells, lint, dust and respiratory droplets.5
Isolation gown standards and critical zones
Are your gowns up to the task? Labeling that shows a product has been tested to and meets appropriate performance standards is one way to determine when to use a particular gown. The American Society for Testing and Materials (ASTM) tests gown performance in the following areas:
- Tensile strength—ASTM D5034, ASTM D1682
- Tear resistance—ASTM D5587 (woven), ASTM D5587 (nonwoven), ASTM D1424
- Seam strength—ASTM D751 (stretch woven or knit)
- Water vapor transmission (breathability)—ASTM F1868 Part B, ASTM D6701 (nonwoven), ASTM D737-75
Unlike surgical gowns, whose critical zones of protection primarily involve the front of a person, isolation gown critical zones cover front and back. The entire gown, including seams but excluding cuff, hems and bindings, is required to have a barrier performance of at least Level 1.6
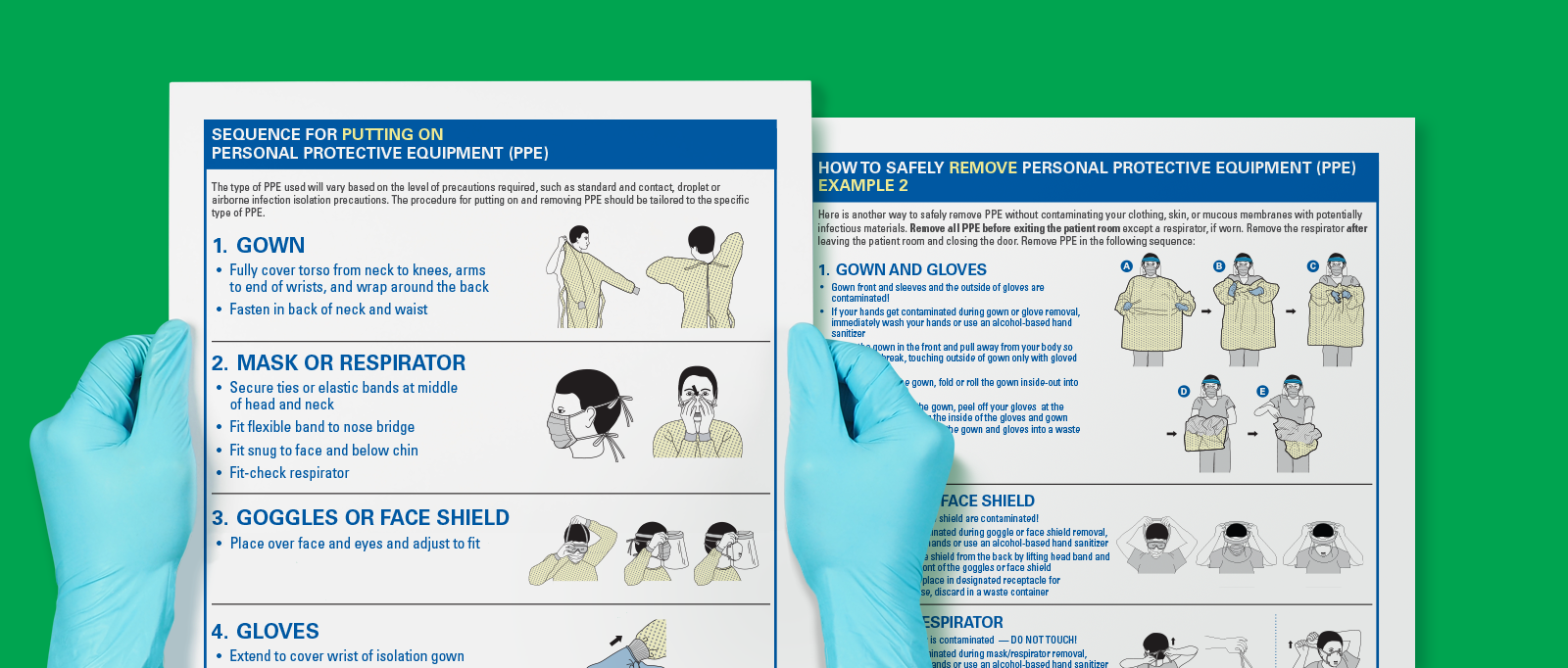
Putting on/taking off isolation gowns
PPE should be put on and taken off in a specific order in a specific way, as shown in the two CDC posters.7 To stay safe, remember that PPE is contaminated when you remove it and should be taken off away from your body. Posters like these can be used to educate staff to ensure that everyone is protected.
Know your options
Isolation gowns are available in different styles and materials. There are gowns that tie at the neck, over-the-head gowns, side-tie gowns and wrap-around (three-hole) gowns, among others. In addition, some gowns are disposable while others are washable. Each style has its ideal uses; you and your team can decide which ones work best in your facility.
Other factors to consider
In addition to barrier resistance properties and other factors discussed above, consider looking at other critical characteristics of protective clothing in your decision-making process:
- Compliance with regulatory agencies
- Durability (abrasion resistance, tensile strength, seam strength)
- Comfort (breathability, air permeability)
- Flammability
- Electrostatic properties
- Cost
- Availability
- Ergonomics/human factors
- Integration with other types of PPE
It’s especially important to understand how the selected gown will work with other items of PPE, including gloves with the sleeve of the gown and face/eye or respiratory protective equipment with the hood or collar area of the gown. These borders are essential to the care provider’s overall safety, because it’s the ensemble of PPE that provides the protection.
In selecting gowns, also consider the physical characteristics of the work environment. Different physical conditions where gowns are used can compromise their material and properties of seam barriers. Certain actions, including kneeling or leaning on a chair or table contaminated with blood, can result in pressure levels that exceed the levels used in the standard test methods. The gowns may no longer provide expected levels of protection under these conditions.8
Key takeaway
Understanding how to choose the right isolation gowns for your healthcare facility can be challenging. Once you know how to assess risk and needs, learn what AAMI levels mean, discover available options and are aware of all the factors to consider, you’ll be well positioned to make the best selections for your frontline staff and prevent the transmission of HAIs.
References:
- FDA. Personal Protective Equipment for Infection Control/Medical Gowns. Available at: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns#g4. Accessed January 5, 2022.
- CDC. NIOSH Personal Protective Equipment Information (PPE-Info). cdc.gov. Retrieved November 11, 2022, from https://www.cdc.gov/PPEInfo/Standards/Info/ANSI/AAMIPB70Class3
- Kilinc, Balci. How well are you protected? What healthcare workers need to know about gown standards and selection considerations. November 2018 CDC presentation. Available at: https://www.health.state.mn.us/facilities/patientsafety/infectioncontrol/ppe/ppewebinar.pdf
- CDC. Implementation of PPE in Nursing Homes. Available at: Implementation of Personal Protective Equipment (PPE) in Nursing Homes to Prevent Spread of Novel or Targeted Multidrug-resistant Organisms (MDROs) | HAI | CDC. Accessed January 12, 2022.
- CDC. The National Personal Protective Technology Laboratory/Considerations for Selecting Protective Clothing. Available at: Considerations for Selecting Protective Clothing | NPPTL | NIOSH | CDC. Accessed January 5, 2022.
- FDA. Personal Protective Equipment for Infection Control/Medical Gowns. Available at: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns#g4. Accessed January 5, 2022.
- CDC. Available at: https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf. Accessed January 27, 2022.
- CDC. NIOSH/NPPTL. Considerations for Selecting Protective Clothing Used in Healthcare for Protection Against Microorganisms in Blood and Body Fluids. Available at: https://www.cdc.gov/niosh/npptl/topics/protectiveclothing/. Accessed December 22, 2021.