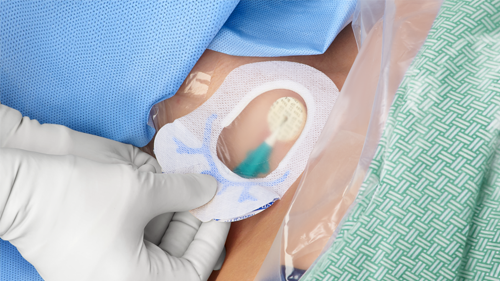
Changing a central line dressing: Standardize your products
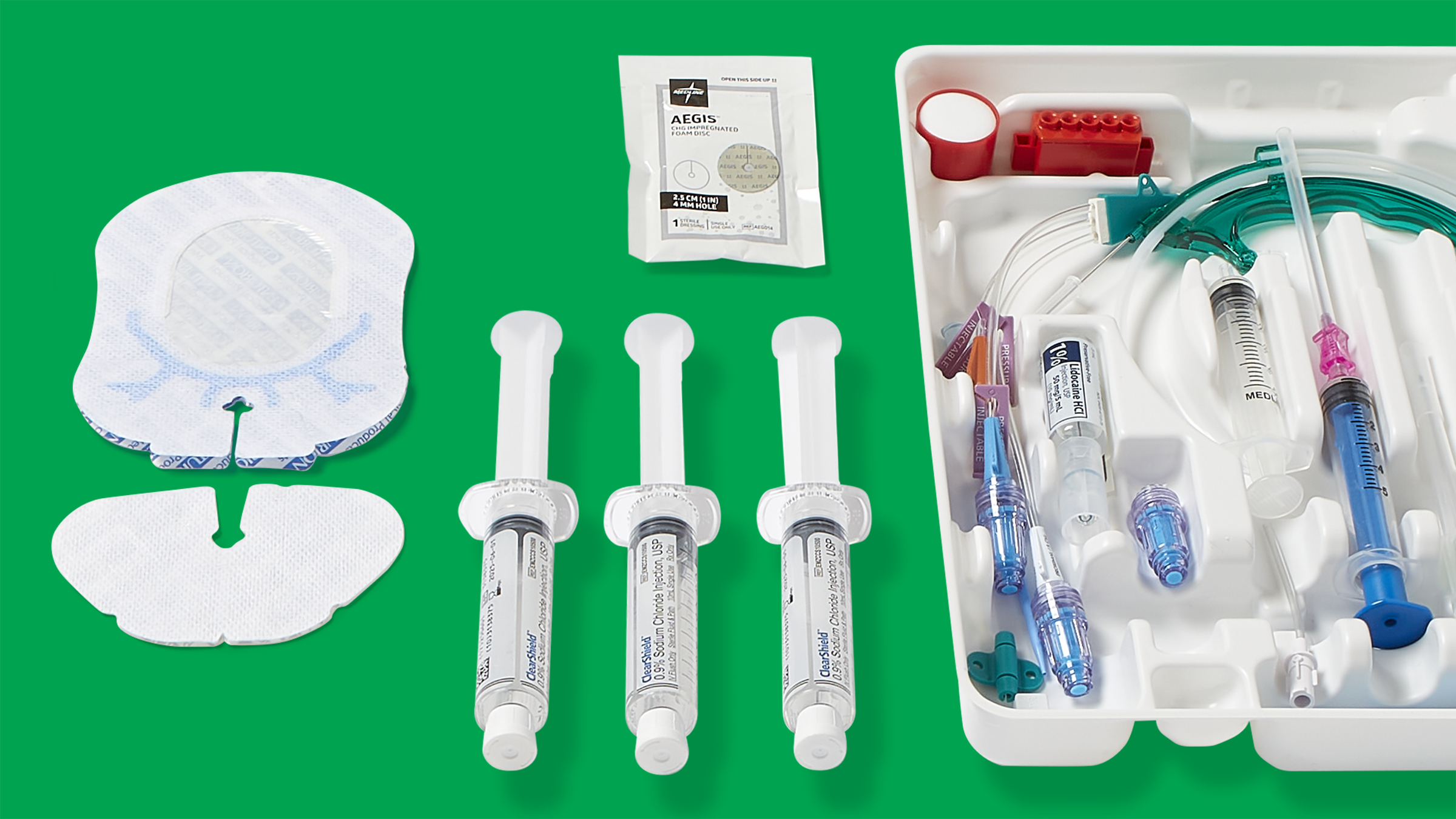
What’s in your CVC insertion and maintenance kits?
When a patient needs a central line inserted, it’s not an easy task. Neither is maintaining the line. In addition to ensuring the central venous catheter (CVC) is doing its job, clinicians monitor for device necessity and dislodgement. And then there’s the dressing, which should remain intact for seven days. When a CVC is left in too long or becomes dislodged—or a dressing becomes disrupted, the potential for central line-associated bloodstream infection (CLABSI) or other complications increases.
With a mortality rate of 12 to 25%, CLABSIs cause the highest number of preventable deaths of all the healthcare-associated infections (HAIs). But the good news is that by implementing evidence-based practices, 65 to 70% of CLABSIs can be prevented.1
How do you ensure everyone follows best practices? By standardizing protocols, processes and products. Let’s examine what’s involved in standardizing CVC dressings and why it’s so important to preventing CLABSIs.
Benefits of dressing standardization
“Standardizing dressings and products when caring for CVC devices allows any clinician who is applying, assessing or changing the dressing to follow the same practice,” says Donna Matocha, DNP, MSN, VA-BC, Medline Manager of Clinical Resources – Acute Care. “When you deviate from standardized products and practices, you end up with variability. You can have lapses in protocol and process that can result in contamination. Or lines can be loosened and the catheter can become dislodged.”
If you are applying a dressing that’s not an approved standard product, the next nurse coming in may not have been trained on how to remove it. Standardized products and processes make it easier to educate and train people, whether new hires or travel nurses, according to Matocha.
In addition, dressings are only one component of CVC insertion and maintenance, so they should be used as part of a kit.
“Using CVC kits promotes continuity of practice across the continuum,” says Vincent Tessitore, VA-BC, Medline Sales Specialist, Vascular Access. “So it’s important that your insertion kit and maintenance kit have the same dressing to avoid variability.”
67%
of CVC dressing changes are unscheduled due to disruptions2
And from a financial perspective, product standardization produces new efficiencies and cost savings. Using common products and kit components enables hospitals to leverage volume purchasing power and potentially earn more advantageous rebate tiers—all of which can lead to lower product costs. Product standardization also decreases SKU count, minimizes waste and reduces inventory on the shelf, which improves efficiency and contributes to cost savings.3
Factors to consider for dressing standardization
Catheter type and insertion site
You need to have the right size to fit the line, so the type of catheter and insertion location are the two main factors.
“You don’t want it too big or too small,” says Matocha. “Insertion site selection deals with the anatomy you have to work around. Are you working around hair? In the case of the groin, are you working around urine and stool? With the neck, are you working with secretions and sweating by intubated patients?”
Then you move to the next area: the patient’s mental and physical shape.
“Determine if the patient is moving around and tugging on the line,” says Matocha. “That’s important on a case-by-case basis with the patient. You wouldn’t standardize based on that, but you have to be able to meet the special needs of different populations. For example, elderly and oncology patients often have fragile skin. And skin is our best defense against infection. That’s why we add skin protection.”
Dressing types
Following are the two main types of dressings for CVCs:
Transparent dressings include flat films or those with cloth borders. Bordered dressings may promote improved adherence of the edges with less lifting.4 Transparent film dressings help minimize the risk of extraluminal catheter contamination; are permeable to water, vapor and oxygen; impermeable to microorganisms; permit easy visual assessment of site; and allow precise dressing placement.
According to the Infusion Nurses Society, transparent dressings should not be considered the single source of securement. Instead, they should be used in combination with other forms of securement to reduce all complications.4
Integrated dressings combine barrier protection, catheter securement and site visibility that enables monitoring of the CVC and insertion site. Security is provided via an integrated anchor that’s built into the product, helping disperse tugging forces from reaching the insertion site. An integrated product helps make the dressing removal process a lot simpler and more comfortable for patients, some of whom have fragile skin.
Special training is needed to ensure clinicians use the products correctly.
Regardless of which type of dressing is used, CHG-impregnated discs are used to provide protection from bacterial growth around the insertion site, allowing continuous protection between dressing changes.
“Your dressing selection should be based on scientific data that proves what is better for patient outcomes.”

Vincent Tessitore, VA-BC
Medline Sales Specialist, Vascular Access
Assess your facility and patient needs
You’ll want to answer key questions before choosing dressing products. For example:
- Walk around your facility. How many CVCs have dressings with loose edges, blood under them or are dirty? How many patients have skin irritation or injury from dressing/tape use or misuse? How many patients have pressure tugging on the device from unsecured tubing? How many CVCs appear to be well secured? Are clinicians following best practices when inserting and maintaining devices? You can use the data to justify more expensive, clinically differentiated dressing and securement options that actually save money in the long run through reduced infections and other complications.
- Does your patient population have special needs? What are they? For example, age, skin integrity issues, insertion site drainage, and comorbidities that affect patient movement.
- Do your vascular access clinicians have preferences? Clinician preference can help give you an idea of what products they might be more inclined to accept as being part of standardized dressings. Ask the clinicians as a team to evaluate current and proposed products against product attributes that drive desired patient outcomes.
- Are their clinicians at your facility who are considered “experts” by their peers? Respected clinical leaders can serve as champions, helping you stand by product decisions based on evidence and clinical practice, not brand preference.
- Does your facility have a contract with a certain manufacturer or distributor? Can you change to a clinically differentiated product?
“Your dressing selection should be based on scientific data that proves what is better for patient outcomes,” says Tessitore. “It’s important to work with and educate supply chain, but decisions should follow recommendations by vascular access experts who know what’s working at the bedside.”
65 to 70%
of CLABSIs can be prevented1
Track what’s working and what isn’t
Once you’ve implemented standardized dressings as part of your insertion and maintenance kits, regularly collect data to measure the impact of the standardization.
- Infection preventionist: How many CLABSI patients had premature dressing changes?
- Electronic medical record: What does the reinforcement documentation say?
- How many dressings are changed before day 5? Day 7?
- How many are reinforced at the time of removal?
- Prevalence rounds: Review dressings on all units: How many are fully intact, lifted, reinforced, revealing insertion site?5
Use this data to identify root causes when things have gone wrong. For example, are clinicians following protocols and processes?
“And sometimes we’re very quick to blame a product, when it’s actually how the product is being used that is causing it to be less effective, says Tessitore. “So you need to continually assess the data to understand what isn’t working and why.”
Then take the lessons learned to modify your standardization.
“The first one may not be effective”, says Tessitore. “Remember, it’s your kit. It can be changed. When you’re modifying, that’s not failure. It’s never one and done in healthcare.”
Key takeaway
CLABSIs cause the highest number of preventable deaths of all HAIs, but 65 to 70% of cases are preventable.1 One way to prevent infection is to standardize products and processes. Taking the appropriate steps to standardize CVC dressings as part of your insertion and maintenance kits helps prevent CLABSIs, increase patient safety and improve product cost-effectiveness.
References:
- Jones, K. Maintenance and removal of central venous catheters. CDC. CLABSI 104 (cdc.gov)
- Timsit, J., Bouadma, L., Ruckly, S., et al. (2012, June). Dressing disruption is a major risk factor for catheter-associated infections. Critical Care Medicine, 40(6),1707-14. https://pubmed.ncbi.nlm.nih.gov/22488003/
- Becker’s Hospital Review. (2019, April 29). Value in product and process standardization. https://www.beckershospitalreview.com/supply-chain/value-in-product-and-process-standardization.html
- Moureau, N. (ed.). (2019). Vessel health and preservation: the right approach for vascular access.
- DeVries, M. Addressing the dressing. (2018). https://cdn.ymaws.com/www.avainfo.org/resource/group/aefeb81a-02aa-4226-80f7-374fd98d34b0/presentations/addressing_the_dressing_2018.pdf