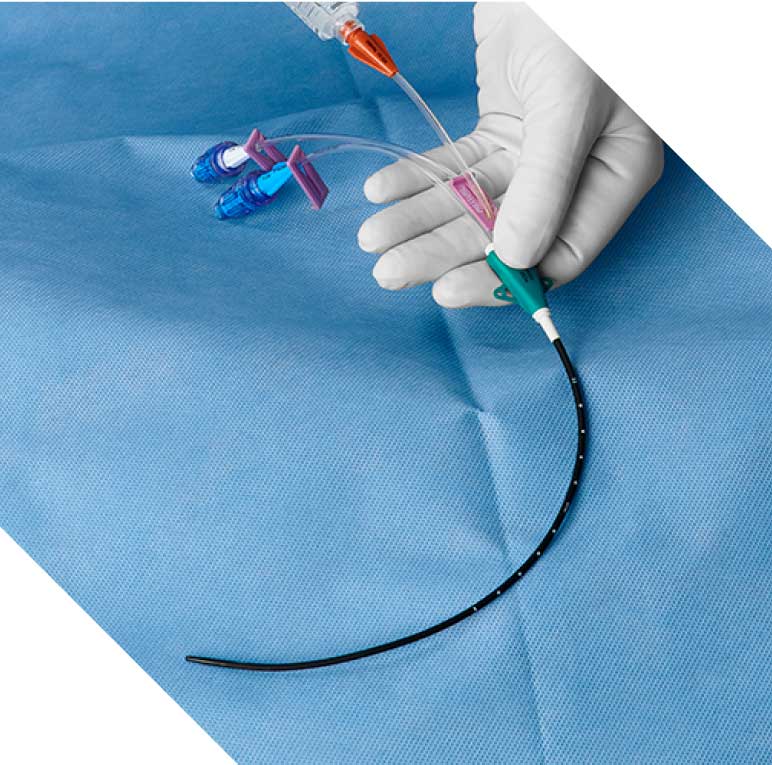
ASA recommends antimicrobial CVCs to reduce CLABSI risk
The 2020 ASA Practice Guidelines for CVC Insertion recommend the use of silver/platinum/carbon-impregnated catheters over uncoated catheters for most effective CLABSI risk reduction.1 The Vantex CVC meets ASA guidelines using time-release silver ions extruded throughout the body of the catheter, internally and externally, to decrease risk of catheter-related blood stream infection (CRBSI).
Vantex helps reduce CLABSI and more—better than other CVCs
CLABSIs occur in 3 to 7% of CVC placements and cost up to $45,000 per occurrence.2,3 Studies demonstrate the Vantex® CVC’s efficacy at reducing and preventing CLABSIs.4,5,6
Outperforms uncoated CVCs
Vantex prevented more bacterial colonization and CLABSIs than polyurethane CVCs.5
Outperforms chlorhexidine-silver sulfadiazine catheters
Pairing both with education, Vantex outperformed silver CHG coated catheters.4 Adding a sterile barrier kit and CHG prep, Vantex reduced CLABSI to 1.6/1000 line days.
Eliminates sulfa allergy risk
Vantex’ extruded silver/carbon/platinum composition eliminates anaphylactic shock risk associated with sulfa in chlorhexidine- silver sulfadiazine catheters.
Vantex CVCs improve patient safety and streamline clinician workflow
Proven technology
Vantex has more than 20 years of proven clinical efficacy.7
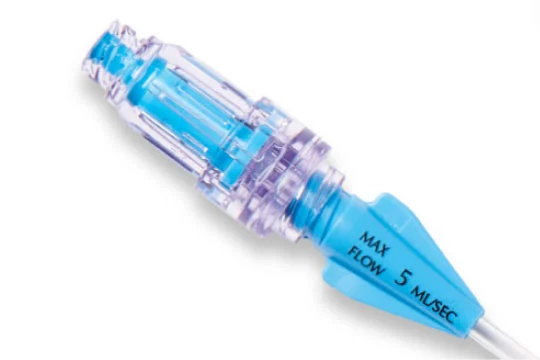
Ease of use
Pre-attached needleless valves save clinicians time.

Less risk of injury
A soft tip reduces the risk of vessel perforation.8
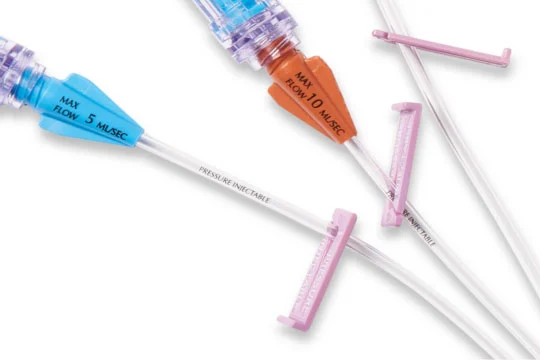
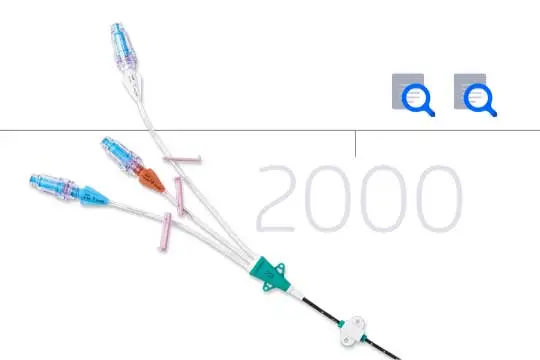
Intuitive design
Clear extension lumens enable better fluid path visualization. Color coded hubs and lumen labels make identification easy.
Experience the Vantex CVC: Request a bundle sample
Please complete and submit the form and a Medline specialist will arrange your insertion bundle sample including a Vantex CVC.
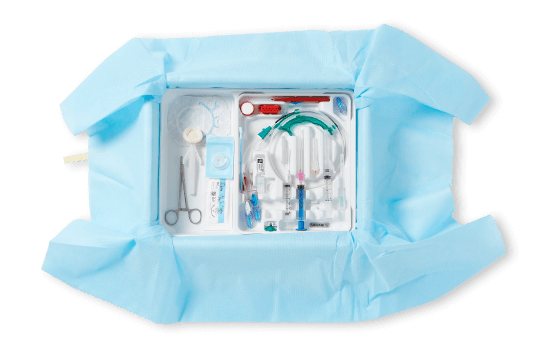
Explore how bundles help improve CLABSI prevention
Set the CVC line up for success from insertion. Our intuitively designed insertion bundle provides clinicians with every supply they need at the bedside. The sequenced tray guides the procedure step-by-step supporting consistent best practice, aseptic technique, efficiency and patient safety.
References:
- Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology January 2020, Vol. 132, 8–43. https://pubs.asahq.org/anesthesiology/article/132/1/8/108838/Practice-Guidelines-for-Central-Venous-Access. Accessed March 1, 2022
- Darouiche RO. Device- Associated Infections: A Macroproblem that Starts with Microadherence. Clinical Infectious Disease. 2001;33:1567-1572.
- Zimlichman E, Henderson D, Tamir O et al. Health care- associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046.
- Ranucci M, et al. Impact of oligon central venous catheters on catheter colonization and catheter-related bloodstream infection. Crit Care Med 2003;31(1):52-59.
- Corral L, Ibanez-Nolla J, Leon M et al. A prospective, randomized study in critically ill patients using the Oligon™ Vantex® catheter. J of Hosp Infection. 2003;55:212-219.
- Fraenkel D, Rickard C, Thomas P et al. A prospective, randomized trial of rifampicin-minocycline coated and silver-platinum-carbon-impregnated central venous catheters. Crit Care Med. Article in Press. 2006; 34(3): 668-675.
- Data on file.
- Gravenstien N, Blackshear RH. In Vitro Evaluation of Relative Performance of Central Venous Catheters: Comparison of Materials, Selected Models, Number of Lumens, and Angles of Incidence to Simulate Membrane. J Clin Monit. 7:1-6:1991.
- Giles, A., Foushee, J., Lantz, E., & Gumina, G. (2019). Sulfonamide Allergies. Pharmacy (Basel, Switzerland), 7(3), 132. https://doi.org/10.3390/pharmacy7030132