Aegis® CHG-Impregnated Foam Disc
Fight CLABSIs from day 1 through day 7
Central line-associated bloodstream infections (CLABSIs) can cause serious clinical and financial burdens for caregivers and patients. Dressing changes are one of three major areas where infection can occur.
What’s causing this? In most cases, skin bacteria at the insertion site. Evidence-based best practice guidelines recommend covering central line catheters with chlorhexidine gluconate (CHG) dressings to help prevent CLABSIs.1
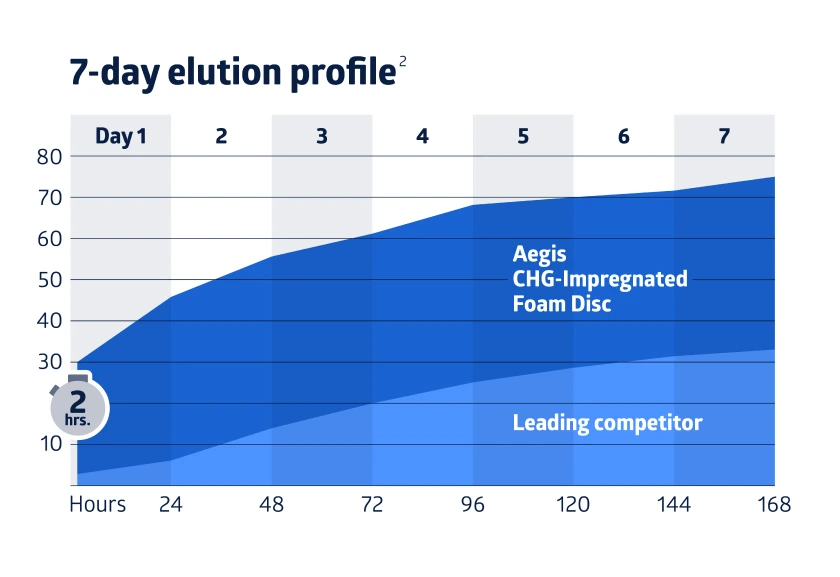
Stop CLABSIs with confidence and protect patients longer. Choose our Aegis foam dressings impregnated with chlorhexedine-gluconate (CHG) and fight bacteria for up to 7 days.2
60% of CRBSIs, including CLABSI
orginate in skin bacteria3
65–70% of CLABSIs
are preventable with current evidence-based practices4
Why clinicians trust Aegis
Based on our in vitro testing, this protective CHG-impregnated foam dressing is proven to help reduce the risk of CLABSIs.2
- Begins killing bacteria within 2 hours
- Sustains progressive “kill” over 7 days
- Provides 360° protection around the insertion site
- Offers the same level of antibacterial effectiveness at day 7 as day 1
- Absorbs 8x its weight in exudate
“We made several recent changes to reduce CLABSIs such as switching to the Aegis patch. We should have made the switch a long time ago.”
Dewayne S. Whitener
CPHC, ASQ–CSSBB, CQE, CQA, CMQ/OE
Kill bacteria fast and help keep patients safe longer
The CHG present in Aegis acts quickly, demonstrating a 4-log reduction in bacteria on the dressing’s surface within 2 hours.2 The prolonged release of CHG provides protection from bacterial growth around the insertion site for 7 days, allowing continuous protection between dressing changes.2
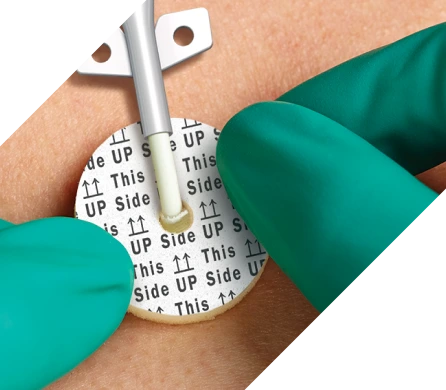
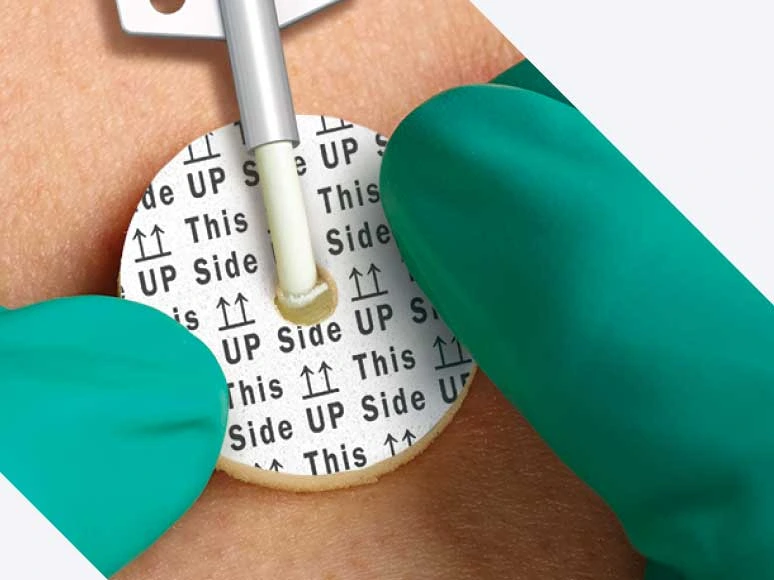
Intuitive design helps ensure accurate dressing placement
Aegis is designed to ensure accurate placement every time for every patient.*
- Radial slit allows easy placement around the catheter
- “This side up” indicators ensure correct application
- Available in two diameters and three different size access holes to fit a wide range of catheters
Experience Aegis for yourself: Request a sample
Simply complete this form and a Medline representative will contact you soon to coordinate your no-cost sample.

References:
- Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O’Grady NP, Pettis AM, Rupp ME, Sandora T, Maragakis LL, Yokoe DS. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infection Control and Hospital Epidemiology, Vol. 35, No. 7 (July 2014), pp. 753-771. Available at https://www.jstor.org/stable/10.1086/676533. Accessed January 18, 2022.
- Data available upon request.
- Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. International Journal of Critical Illness & Injury Science. 2014;4(2):162-167. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4093967/?report=printable. Accessed January 18, 2022.
- Septimus EJ, Moody J. Prevention of Device-Related Healthcare-Associated Infections. F1000Res. 2016 Jan 14;5:F1000 Faculty Rev-65. doi: 10.12688/f1000research.7493.1. PMID: 26918162; PMCID: PMC4754053.
*CHG-impregnated foam dressings should not be used on premature infants or patients with a known sensitivity to chlorhexedreine gluconate.