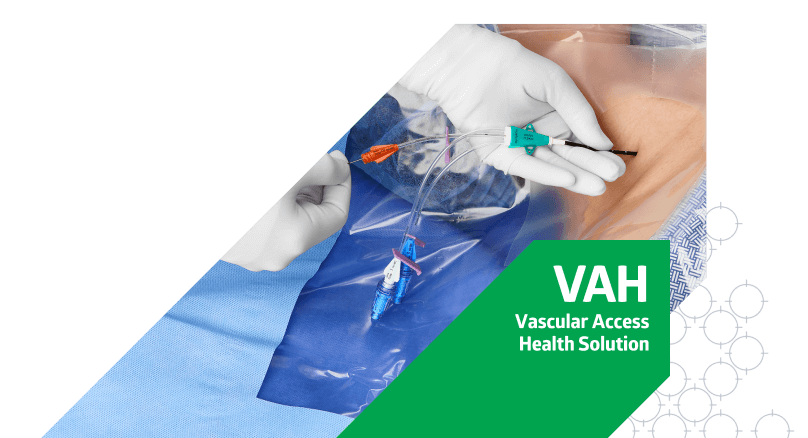
Bundle up to help prevent CLABSIs
Central line-associated bloodstream infections (CLABSI) lead to thousands of deaths every year and cost billions of dollars to the U.S. healthcare system.1 Evidence supported by every major health agency shows that using a bundle strategy as part of your CLABSI prevention plan can effectively reduce CLABSI rates.2,3,4,5
Key bundle components include:
• Hand hygiene
• Maximum barrier precautions during insertion
• Skin cleansing with alcohol-based chlorhexidine
• Optimal catheter site selection
• Daily review of line necessity with prompt removal
• Insertion checklist
Our intuitively designed CVC insertion bundle provides a systematic approach to CLABSI prevention. It contains everything your clinicians need in the right sequence to minimize variation, enhance clinical workflow and prevent infection.
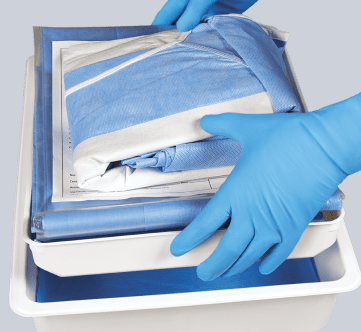
Maximize procedure safety and efficiency with a customized bundle
Our customizable bundle addresses each step in the venous catheter insertion process and specific objectives that must be met to safeguard against infection.
Click the icons to explore
Protect your patient and insertion team
Equips your clinicians with everything they need to help prevent cross contamination.
Protect your patient and insertion team
Equips your clinicians with everything they need to help prevent cross contamination.
Streamline the insertion process
Anticipates the inserter’s needs, letting the team focus on the patient rather than supplies. All components are sequenced in order of use, reducing practice variation and optimizing the entire insertion process.
Streamline the insertion process
Anticipates the inserter’s needs, letting the team focus on the patient rather than supplies. All components are sequenced in order of use, reducing practice variation and optimizing the entire insertion process.
Support post-insertion compliance
Guards the catheter and insertion site from infection and other complications after the procedure by providing stabilization and bacterial protection to help prevent premature dressing disruptions.
Support post-insertion compliance
Guards the catheter and insertion site from infection and other complications after the procedure by providing stabilization and bacterial protection to help prevent premature dressing disruptions.
Request a free utilization review
Building the right CVC bundle for your insertion team begins with a utilization review. Our vascular access clinical experts take a detailed look at your current insertion products and protocols. Next, we analyze our findings and recommend changes to improve standardization and clinical workflow.
Get started today. Simply complete this form and a Medline Representative will be in touch with you shortly.
Make CLABSI prevention second nature
Explore our full Vascular Access Health Solution. From best practice guidance and education to the right system of products, we help you drive a culture of safety across your organization.
References
1. Haddadin Y, Annamaraju P, Regunath H. Central Line Associated Blood Stream Infections. [Updated 2020 Dec 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430891/ Accessed March 9, 2021.
2. Association for Professionals in Infection Control and Epidemiology. Guide to Preventing Central Line-Associated Bloodstream Infections. Washington, DC; APIC Implementation Guides, December 2015. Available at: http://apic.org/Professional-Practice/Implementation-guides/#implementaion-guide-7464 Accessed March 4, 2021.
3. The Joint Commission. Preventing Central Line-Associated Bloodstream Infections: A Global Perspective. Oak Brook, IL: Joint Commission Resources, May 2012. Available at: https://www.jointcommission.org/assets/1/18/CLABSI_Monograph.pdf Accessed March 4, 2021.
4. Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. O’Grady NP, Alexander M, Burns LA, et al. Centers for Disease Control and Prevention website. Available at: https://www.cdc.gov/infectioncontrol/pdf/guidelines/bsi-guidelines-H.pdf Accessed March 4, 2021.
5. Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O’Grady NP, Pettis AM, Rupp ME, Sandora T, Maragakis LL, Yokoe DS. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infection Control and Hospital Epidemiology, Vol. 35, No. 7 (July 2014), pp. 753-771. Available at https://www.jstor.org/stable/10.1086/676533. Accessed March 04, 2021.
*The activity of the antimicrobial agent is localized at the catheter surfaces and is not intended for treatment of systemic infections. In vitro testing demonstrated that the Oligon agent provided broad spectrum effectiveness (≥ 3 log reduction from initial concentration within 48 hours) against the organisms tested: Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, Enterococcus faecalis, Candida albicans, Escherichia coli, Serratia marcescens, Acinetobacter calcoaceticus, Corynebacterium diptheriae, Enterobacter aerogenes, GMRSa, Pseudomonas aeruginosa, Candida glabrata and VRE (Enterococcus faecium).